[ad_1]
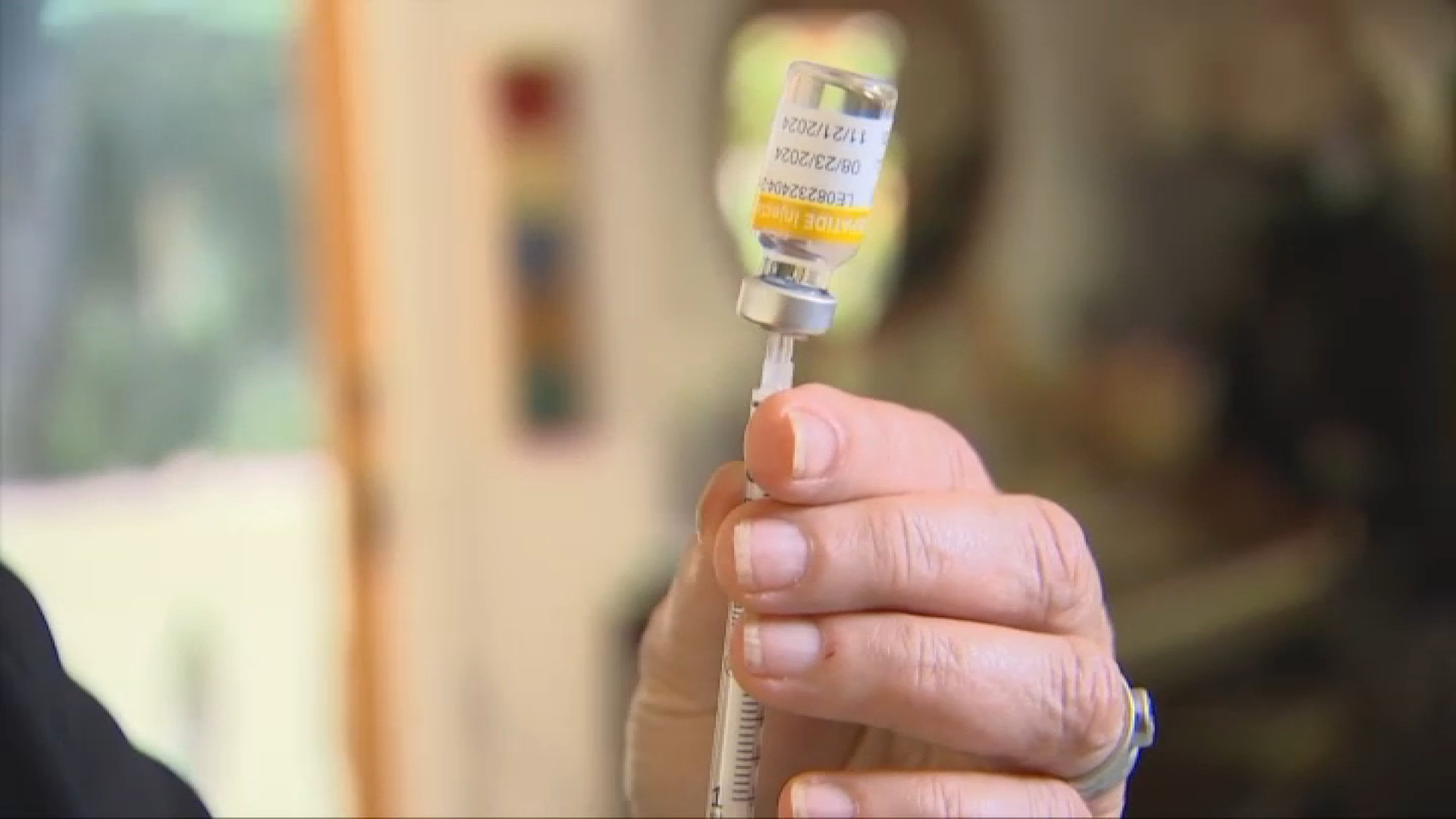
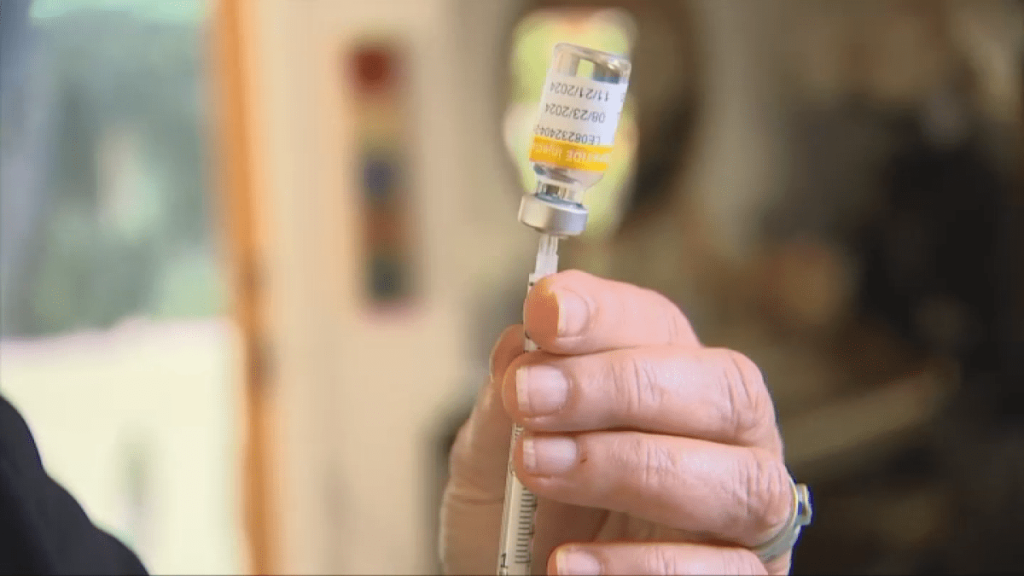
The Food and Drug Administration allows licensed compounding pharmacies to manufacture copies of certain medicines that are in short supply, including the main two FDA-approved diabetes and weight loss drugs. It contained the ingredients semaglutide and tirzepatide.
“I did some research and found what appeared to be a reputable pharmacy through a telemedicine company,” said the patient, who asked to be identified as Lena.
She turned to compounded tirzepatide after insurance coverage for FDA-approved Zepbound was discontinued. Her doctor agreed that a new type of weight loss drug might help her.
“For two years, I went through different types of diets and working on myself to lose weight,” Lena said.
She wanted to see if she could lose weight and control her metabolic problems by continuing to take the medication. After following a diet and exercise regimen, she began to lose stubborn weight. She said blood tests also showed positive changes.
What is happening is the confusion of legal compounding, state-licensed compounding pharmacies with illegal online drug sellers and counterfeit drug manufacturers.
Tenille Davis, Pharmacy Alliance
But she recently received a letter from the telemedicine company she ordered the compounding medication from, stating that they will no longer be able to refill her tirzepatide after Dec. 2, 2024.
In October, the FDA declared the tirzepatide drug shortage “resolved,” even though many patients still reported having problems filling the drug.
“Compounding pharmacies are only allowed to make these copies if they currently lack that status,” said Tenille Davis, Chief Advocacy Officer at the Compounding Pharmacy Alliance. “Thus, once changed to resolved, 503a traditional compounding pharmacies are no longer able to manufacture these copies of tirzepatide injection.”
Davis said that while FDA policy clearly allows for compound drugs to help patients during drug shortages, there are problems.
“What is happening now is the confusion of state-licensed compounding pharmacies that are legally compounding drugs with illegal online drug sellers and counterfeit drug manufacturers.”
In fact, a study recently published in JAMA, the Journal of the American Medical Association, tracked the safety of semaglutide, the main ingredient in FDA-approved Ozempic and Wegovy, and found that it is being used by “illicit online pharmacies to sell products without a prescription.” It turned out that it was on sale. Shipping unregistered counterfeit products. ”
“It’s a real problem. I’ve worked in health as well as emergency medicine, so I’ve seen it firsthand,” said Dr. Tyler McAtee. “I’ve seen patients come in because they’re getting products from overseas or out of state and they don’t really know who the supplier is.”
Now that all of this is happening, I decided to reduce my risk, reduce my anxiety, and go straight to the pharmacy to pick up my Zepbound.
Lena, weight loss patient
Dr. McAtee, who recently opened Ranch Wellness Center in Orange County, prescribes compounded tirzepatide for weight management along with regular patient check-ins and uses a method he calls microdosing to help some patients. For this reason, I have been using combination preparations on and off repeatedly.
“My main motivation was to lose weight to combat cardiovascular disease and reduce my risk,” patient Vic Gondola said. “Our focus is on whether we can maintain this weight with minimal medication.”
Dr. McAtee said he was prepared to reduce Gondola’s dose.
“The idea is to use that as a kind of boost,” he said. “Use it as a way to teach your body how to function more optimally.”
But experts in the field, like Dr. Amanda Velasquez, director of obesity medicine at Cedars-Sinai Center for Weight Management and Metabolic Health, point to scientific research on this new class of weight loss drugs.
“When these drugs work to help the body reprogram how it manages weight, they are only effective while the drugs are still active in the body,” Dr. Velasquez explained. “These drugs don’t just reduce appetite and slow down the gut. It’s not a form of retraining the individual. These drugs aren’t really changing our biology because our biology isn’t working properly. Masu.”
Dr. Velasquez is also concerned about what is actually in the drug’s compounded form and the cycles of patients taking and not taking the drug.
“Clinically speaking, there are concerns about doing it because of what it can cause,” Dr. Velasquez explained. “Are you trying to lose weight by disrupting your body’s set point for what it wants to be at a normal weight? Could it rebound back up and then end up even higher than when you started?”
Confusion between branded and compounded products, as well as access issues, have led many patients to seek cheaper and more available options.
The price of combination preparations can be less than half the price of branded drugs. These are not generic drug options, and medical spas and compounding companies that promise generic versions of branded drugs are being sued by drugmakers Novo Nordisk and Eli Lilly.
However, licensed compounding pharmacists have raised concerns about whether patients have access to safe products.
“What coming out of the tirzepatide shortage has taught us is that we need to be better prepared for when we come out of the semaglutide shortage,” Davis said. “We don’t want patients to go without their medication, so[compounding]pharmacists can take steps like getting a prescription for an FDA-approved product now and putting it on hold.”
But after questioning whether to fill the needle correctly, wondering what was in the combination drug she was injecting, and ultimately worrying about whether she would be able to obtain the drug, Lena turned to pharmaceutical company Eli Lilly. I found the coupon on the website.
“With all this going on, we decided to reduce risk, reduce anxiety, and pick up Zepbound directly at the pharmacy.”
With Eli Lilly’s discount, Lena’s monthly costs would be about $500, which is about $200 more than filling a compound medication. Without insurance, brand name weight loss drugs can cost more than a thousand dollars a month.
Lena also hopes that by sharing her experience, the stigma surrounding these weight loss drugs will be reduced and ultimately insurance companies will recognize the positive health benefits of covering them. I look forward to connecting with you.
Meanwhile, both Eli Lilly and Novo Nordisk recently asked the FDA to ban synthetic copies of their drugs, saying their drugs are too complex to make safely. The FDA is considering this and is currently evaluating whether there is still a shortage of tirzepatide.
Dr. Velasquez, who receives a small fee from Novo Nordisk as a consultant, recommends all weight loss patients talk to their doctors about other FDA-approved weight loss drugs if this new class of drugs is not available. are.
To find out if a compounding pharmacy operates a licensed facility here in California or in another state, the Pharmacy Compounding Alliance has created a search engine.
Lawyers for the Pharmacy Compounding Alliance recently sent this letter to the FDA objecting to Eli Lilly’s designation of tirzepatide on its list of “drugs that present demonstrable compounding difficulties.”
The California Board of Pharmacy also allows consumers to search for the status of specific sterile compounding pharmacies in this state.
[ad_2]Source link