[ad_1]
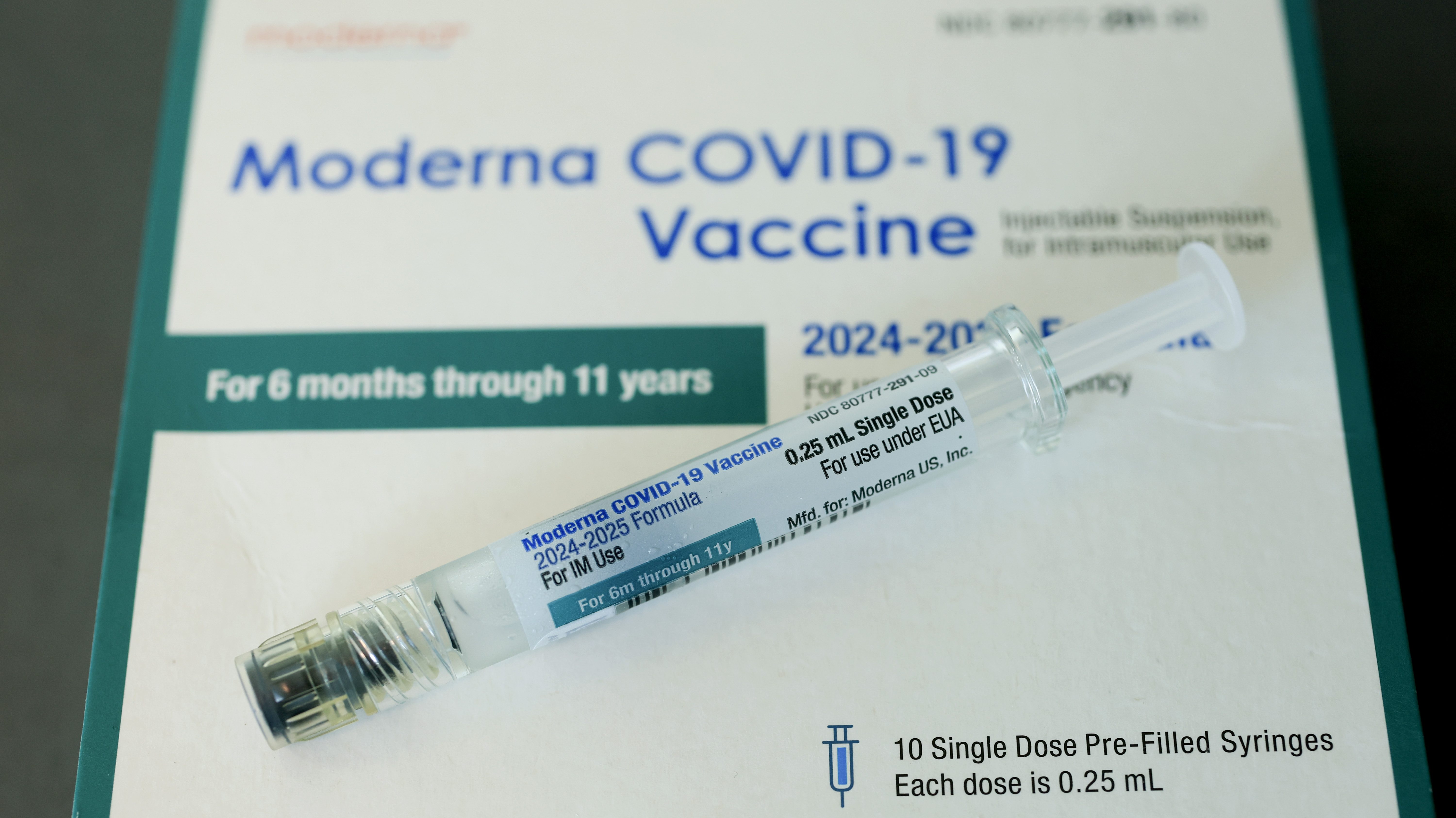
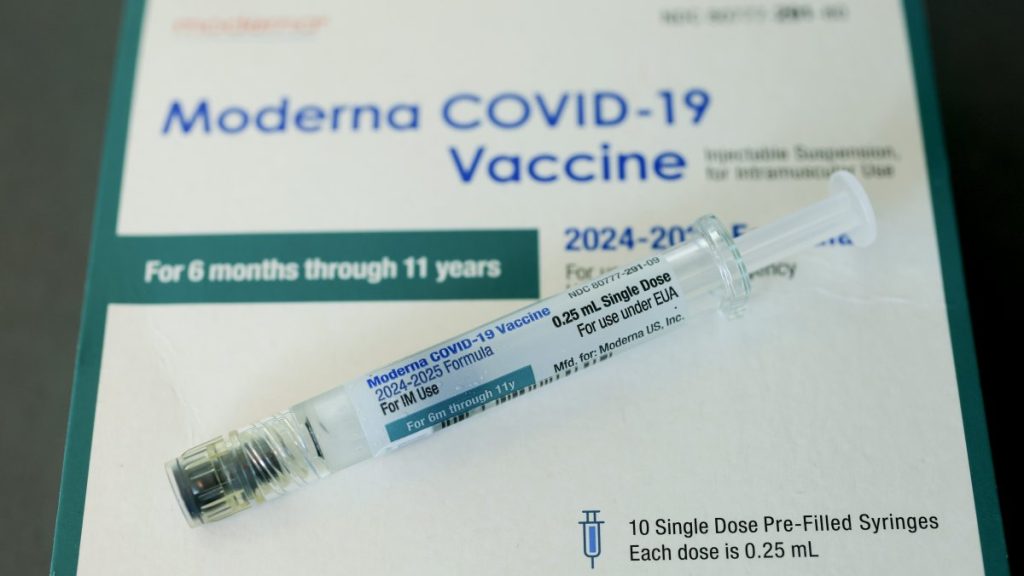
For the first time in 30 years, the American Academy of Pediatrics has effectively diverged from the US government’s vaccine recommendations.
The group’s new Covid-19 recommendations released Tuesday were announced during a turbulent year for public health as vaccine skeptics are in power in the new Trump administration and government leadership is becoming increasingly disrupted.
This would not be useful, Dr. James Campbell, vice-chairman of the AAP Infectious Diseases Committee.
“That would be a bit confusing, but our opinion is that children need to make the right choices to protect them,” he added.
AAP highly recommends Covid-19 shots for children between 6 months and 2 years old. According to AAP, if parents want to get the child vaccinated, shots are also recommended for older children.
That is not the guidance established under US Secretary of Health, Robert F. Kennedy Jr.
Campbell, an infectious disease expert at University of Maryland, said that children aged 6 months to 2 years are at a higher risk of severe illness from Covid-19, and it was important that the recommendations continue to emphasize the need to be vaccinated.
Also, older children with chronic lung disease and other conditions are recommended for vaccinations to older children at higher risk for severe illness, AAP said.
The 95-year-old Itasca-based organization in Illinois has been publishing vaccination recommendations for children since the 1930s. In 1995, we synchronized recommendations and advice from the federal Centers for Disease Control and Prevention.
Since then, there have been some minor differences between AAP and CDC recommendations. For example, AAP advises children to be vaccinated against HPV from the age of nine. The CDC says it’s okay, but emphasizes vaccinations for 11 and 12 years olds.
But this is the first time in 30 years that the recommendations have been different “in an important or substantive way,” Campbell said.
Until recently, the CDC has been urging an annual Covid-19 booster for all Americans over six months following recommendations from infectious disease experts.
However, in May, US Health Secretary Robert F. Kennedy Jr. announced that the Covid-19 vaccine is no longer recommended for healthy children and pregnant women. A few days later, the CDC issued a language that healthy children might take shots, but there were no longer “needed” recommendations.
The idea that healthy older children may be able to skip the Covid-19 booster has been brewed for some time among public health experts. As the Covid-19 pandemic has declined, experts are increasingly debating the possibility of focusing vaccination efforts on people over the age of 65.
The June CDC Expert Panel was set to recommend for fall shots. Among the options the panel was considering were whether they proposed shots of high-risk groups but gave low-risk people the option to get vaccinated.
However, Kennedy also decided to bypass the group, reject a panel of 17 members and appoint a small panel of his own, including vaccine skeptics. Kennedy also later ruled out AAP, the American Medical Association and other top healthcare organizations working with advisors to establish vaccination recommendations.
Kennedy’s new vaccine panel has yet to vote for recommendations for the Covid-19 shot.
The panel continued to recommend and supported fall flu vaccinations, but made a decision that led to another notable difference from AAP.
The new advisory board voted that people would only take the flu vaccine packaged as a single dose and not contain the preservative thimerosal.
The AAP has no evidence of harm from preservatives and the doctor recommends using a licensed influenza vaccine product suitable for patients.
The new variant is called NB.1.8.1. It arrives as the official US stance on Covid-19 vaccination is changing.
[ad_2]Source link